

Still, aside from the exceptions above, all elements have the same molar mass as the atomic masses on the periodic table. phosphorus is normally found in clumps of four atoms, P 4, and sulfur is found in clumps of eight atoms, or S 8. That each molecule of the element has two atoms of that element stuck together.Īs a result, the formula of hydrogen is H 2, nitrogen is N 2, etc. In the case of hydrogen, nitrogen, oxygen,įluorine, chlorine, bromine, and iodine, the element is diatomic, meaning In some cases, the element is usually found in a differentįorm than just one unbonded atom. So, in our example, carbon has a molar mass of 12.01 grams per mole.
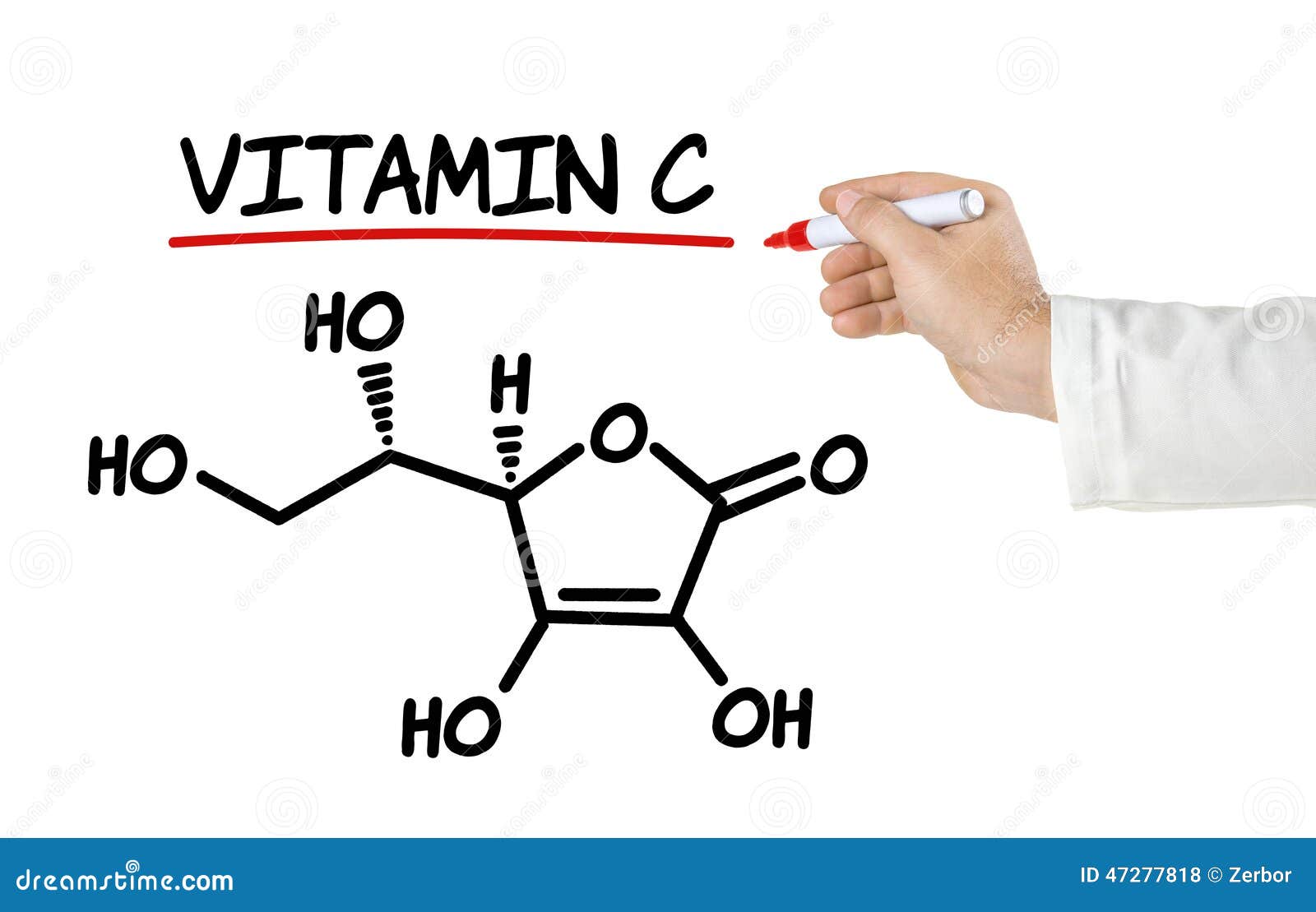
For example, if you want to find the molar mass of carbon, you would find the atomic mass of carbon on the periodic table, and this is equal to the molar mass in grams per mole. The molar mass of elements is found by looking at the atomic mass of the element on the periodic table. How can I find the molar mass of an element? It is also sometimes called: Molecular Mass, Molecular Weight, Formula Mass, or Formula Weight. One atomic mass unit (u) is equal to 1/12 the mass of one atom of carbon-12. If the polyatomic anion contains H -ion, bi - or hydrogen is used as a suffix.Molar mass is the mass (in atomic mass units) of one mole of a of a substance. Example: F – -FlorideĪnions with oxyanion (oxygen + another element) usually have an add-on. Example: NaCl containing Na + (iron ion) and Cl – (non-ferrous ion)Īnions with -1 negative charge usually have a long-suffix.

With a combination of metal and non-metallic, the metal is first named followed by non-metallic. Compounds with more than two elements.Īn atom with a positive charge is called a cation and an atom with a negative charge is called an anion. The following points should be kept in mind while writing a chemical formula. The formula of the radicals and the valency of the elements in that compound. To write a chemical formula, it is important to know the symbol of each elements present in the compound. Empirical formulas can be derived from molecular formulas.Īs the name suggests, the structural formula for the chemical compound provides insight into the arrangement of atoms in a molecule. The empirical formula of glucose is CH 2O. Empirical formulas are usually obtained based on analysis of data. The empirical formula for a given chemical compound represents the ratio of the elements present in that compound. For example - molecular formula of sugar cells is C 6H 12O 6. In the molecular formula, elements are denoted by their symbols (such as the periodic table) and the number of atoms of each element in a molecule is subscript. The molecular formula provides insight into the number of elements present in a compound. The term 'chemical formula' usually refers to the molecular formula of a compound (representing the total number of atoms of each component in a single composite molecule), the composition of chemical compounds can be expressed in a different ways are given below:
CA ELEMENT AND C ELEMENT FORMULA FREE
The chemical formula of the compound is important while representing in the chemical equation.Ĭhemical formulas can also be used to represent ions, free radicals and other types of chemicals. They represent the proportions by which constituent elements combine to form a compound. For example, the chemical formula of water is H 2O, that suggests two hydrogen atoms which combine with one oxygen atom to form a water molecule.Ĭhemical formulas provide insight into the chemical composition of compounds. Chemical formulas provide insight into the elements that constitute the composite molecules and the extent to which the atoms of these elements combine to form such molecules. The chemical formula of a given compound is a symbolic representation of its chemical composition.


 0 kommentar(er)
0 kommentar(er)
